Analysis of residual DNA by high-throughput sequencing (SSV-Seq) in rAAV lots
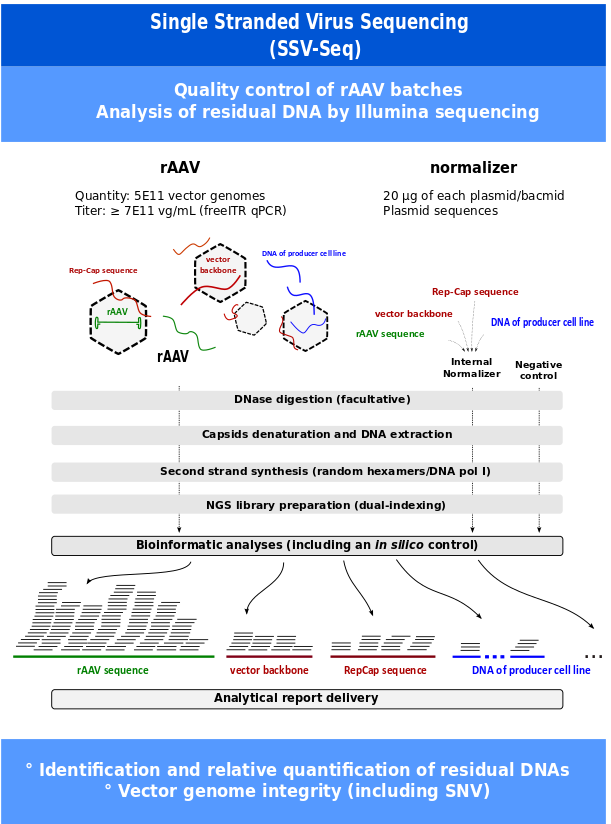
With the success of clinical trials using recombinant adeno-associated viral vectors (rAAV), regulatory agencies ask for a more comprehensive characterization of process- and product- related impurities found in rAAV stocks in order to assess the potential risks for patients. The CPV (translational vector core) has developed a protocol, the Single-Stranded DNA Virus Sequencing (SSV-Seq) 1-3 and a dedicated bioinformatics pipeline in order to :
- Identify and Quantify residual DNAs (relative percentage of each DNA species)
- Analyze rAAV genome identity (including SNV)
The CPV offers to academics and biotech companies to perform this non-selective quality control for a comprehensive analysis of residual DNAs in your purified rAAV batches by high-throughput sequencing.
The CPV quality management system meets the requirements of the Management System Standards ISO 9001:2015.





