Gene Therapy for retinal diseases
Our group works on engineering approaches to solve neurodegeneration with a particular focus on the retina.
- Three axes of research that depend on a new generation of AAV vectors :
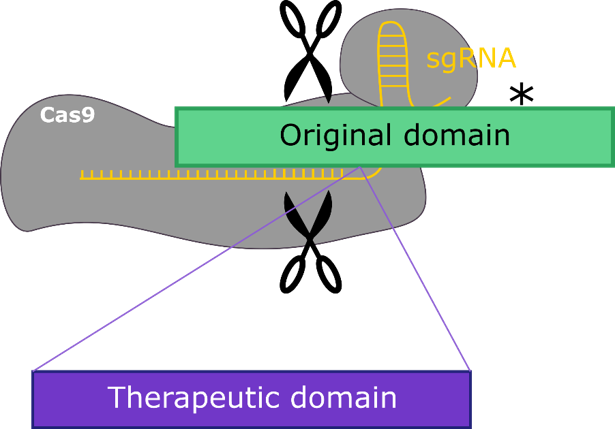
1. Engineering the ATPase domain
Modularity has allowed for the evolution of complex multi-functional genes. The same semi-autonomous functional modules emerge across lineages to enable the function of large proteins such as pumps and transporters.
The nucleotide-binding domains of the ATPase unit is just one very abundant example. We are designing a generic module for correction of ATPase-dependent genes.
Our current target of interest is the ATPase-binding cassette transporter (ABCA4), a causal gene in the juvenile maculopathy, Stargardts disease
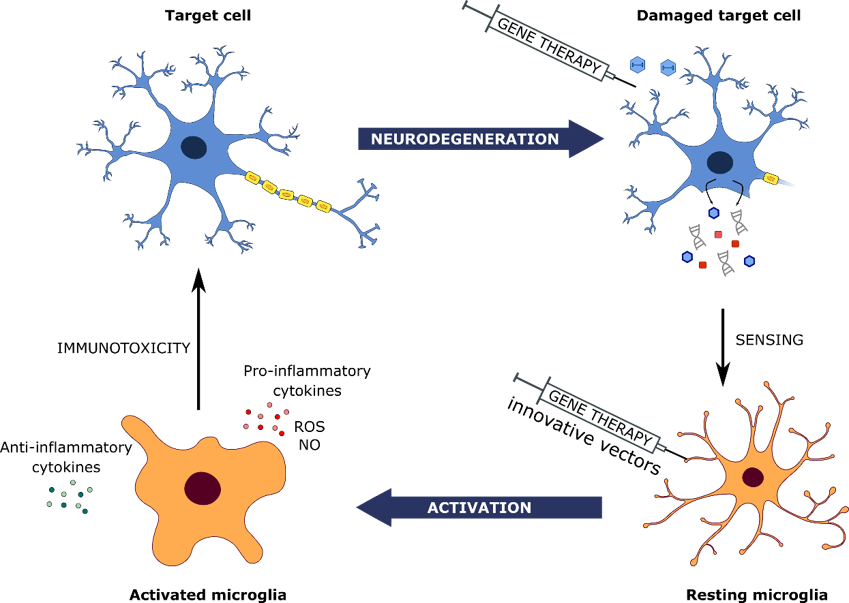
2. Engineering the retinal glia
In inflammatory diseases such as Age-related macular degeneration (AMD) microglial cells have a largely protective role early on in the disease.
We are engineering these cells to restrict expression of checkpoint regulator chemokine genes so as to keep these cells in a protective state and limit disease progression.
Non-activating, chemically modified vectors and rationally designed promoters are essential for targetting immunomodulatory transgene expression in these cells.
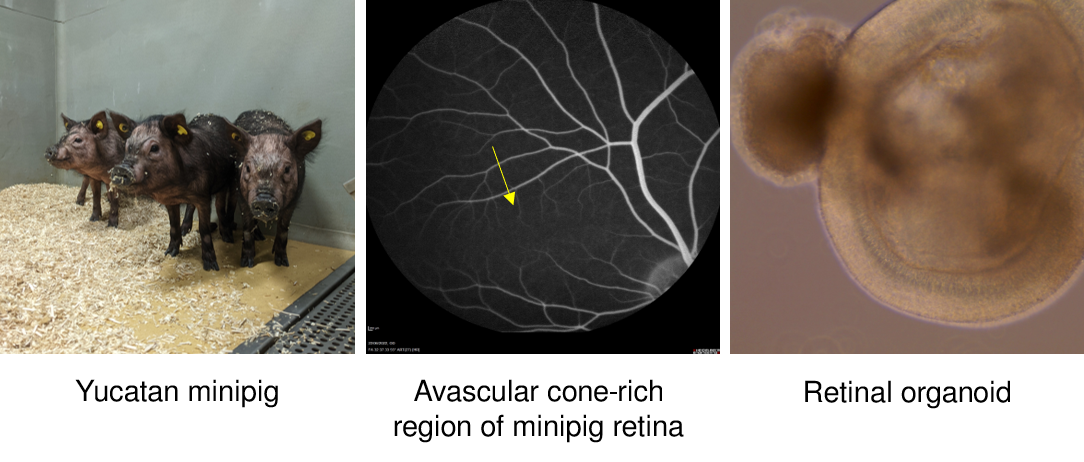
3. Modelling disease states
Rodent models offer enormous insight into the effects of a mutation. However, these models are limited when evaluating therapies targetting the macula, the source of functional human vision. We are using yucatan pig models for AMD research, characterizing end-points that can be used to measure preclinical efficacy of therapeutic candidates.
Our patient retinal organoids allow us to model visual cycle dyanamics in Stargardts disease research, a juvenile form of macular degeneration (In collaboration with Institut de la vision, Paris) .
The vector
Working with the vector innovation group we are testing chemically-modified vectors that broaden the scope of transduction
while shielding the vector from signalling an immune response.
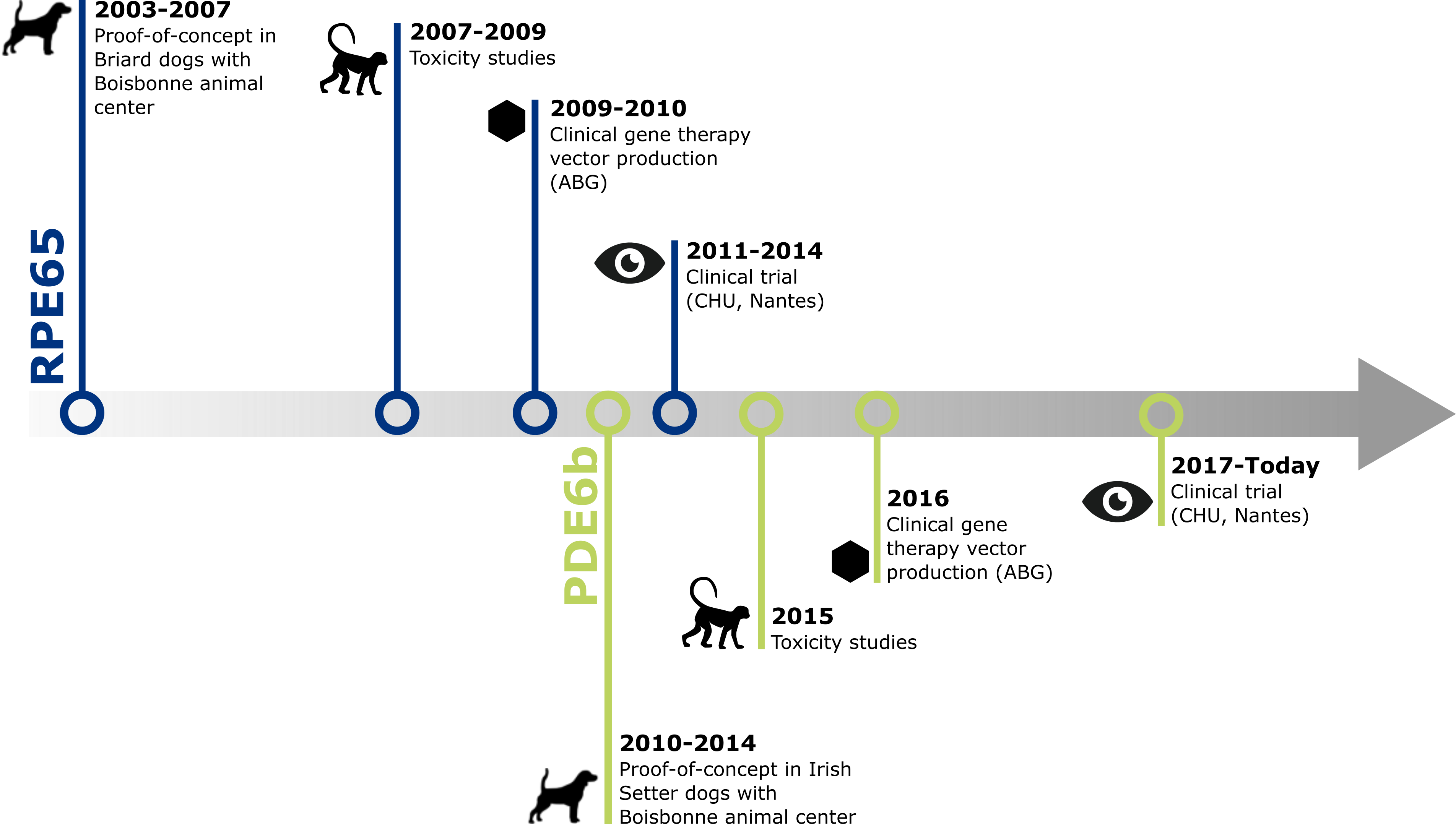
Clinical Proof Of Concept for Gene therapy
The team has extensive experience in the field of translational gene therapy for inherited retinal dystrophies.
In particular, the group has performed preclinical gene therapy P.O.C. for Leber's amaurosis related to RPE65 protein deficiency and PDE6β retinal dystrophy enable development of two Phase I/II clinical trials.

to support one of its research programmes.
Team members
- Oumeya Adjali, MD, PhD, DR2 INSERM, Head of the lab
- Bongnam Niaudet Jung, PhD, Senior Scientist
- Sébastien Paillusson, PhD, Senior Scientist
- Allwyn Pereira, PhD, Senior Scientist
- Joanna Demilly, Engineer
- Sarah Renault, Engineer
- Estelle Toublanc, Engineer
- Ragnar Gautier, Engineer
- Clément Morival, Doctoral student
- Elodie Mortier, Assistant Engineer
CLINICAL:
- Pr Guylène Le Meur, MD, PhD
- Dr Jean-Baptiste Ducloyer, MD, PhD
- Lyse Libeau, Orthoptist
- Fabienne Rolling, PhD
- Baptiste Ameline, PhD
- Kizito Tshilenge, PhD
- Carolina Isiegas, PhD
- Juliette Varin, PhD
- Therese Cronin, PhD,
- Pr Michel Weber, MD
- Alexandra Mendes-Medeira, Tc
- Nathalie Provost, Tc
- Noémien Maillard, Ms
- Minja Velimirovic, Ms
- Jean-Baptiste Deltour, Ms
- Betty Dupas, Ms