Vector Core
The vector core facility is dedicated to the production of research/pre-clinical grade viral vectors
Our team offers end-to-end services for your vector :

Selection
- rAAV
- rAd
- rLV
Design
- Custom design
- Serotype
- Promoter
- Transgene
- Codon optimization
- Cloning
- Plasmid production
Production
- Research grade production
- Formulation
We produce viral vectors which are used in (proof of concepts, etc.) and preclinical studies.
Our Vector core is your ideal partner since over 25 years, and with more than 150 batches (~400 CS5) per year,
our scientific experts have been guiding you in the design of your recombinant viral vectors,
and offering you products tailored to your needs and objectives.
Focus on rAAV vector production and serotypes produced by ViVeM center :
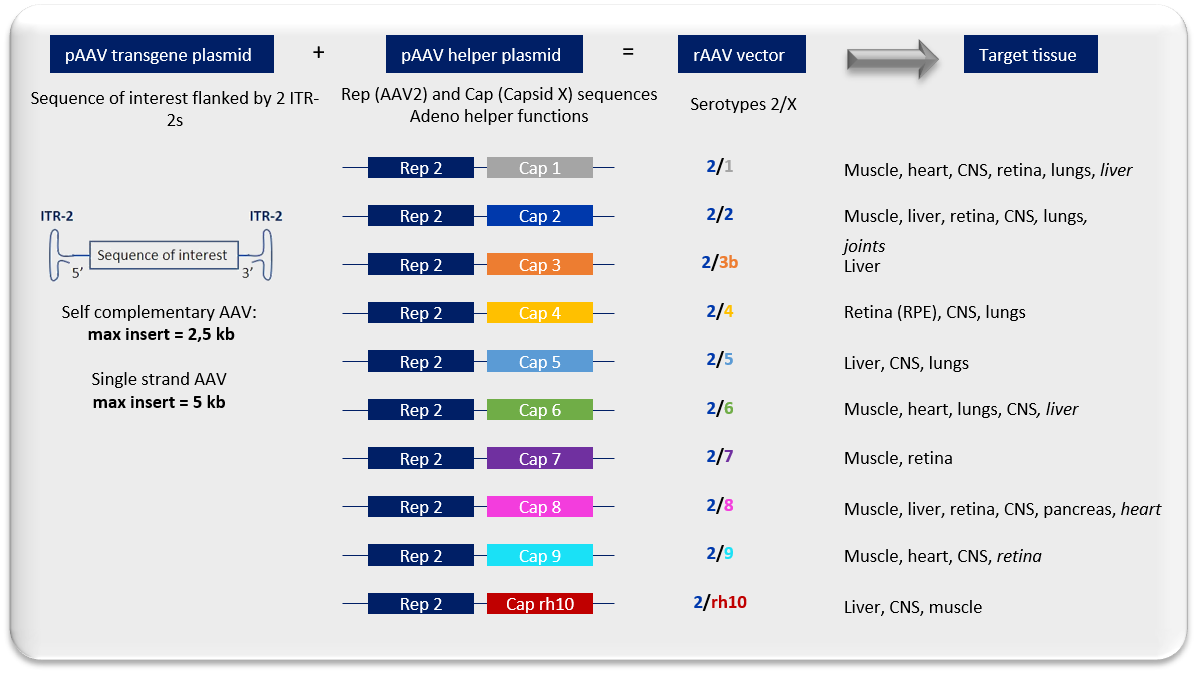
Wild type AAVs and variants (PHPeB, PHP.S, Anc80, MacPNS1, DJ, 2i8 ...) can be produced upon client request.
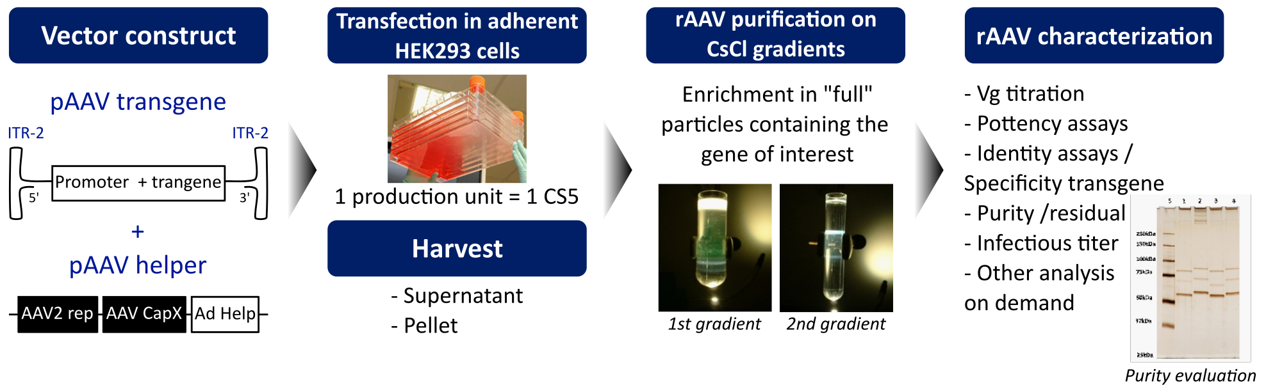
Our production process for research grade rAAVs :
Updated on 01 October 2024.