ChemICALS
Chemistry for Innovative bioConjugation And Labeling Strategies
AAVs are efficient therapeutic platforms for the treatment of genetic diseases. Yet, past and ongoing clinical trials have highlighted some limitations, including the need for high doses to achieve therapeutic benefit, and the off-target transduction of various tissues.
Our group focuses on the development of novel chemically modified AAV particles with increased therapeutic effect, at the interface between chemistry and vectorology (Mathieu Mével). Chemically modified AAVs should enable to greatly decrease off-target effects, hence maximizing transduction in tissues of interest and improving the therapeutic index in AAV-based gene therapy. The chemically modified AAVs are generated through covalent coupling of tissue-specific ligands on the surface exposed residues of the AAV capsid. The main advantage of this chemistry oriented approach is to be able to functionalize AAV particles with various ligands which could not be genetically encoded, including polymers, sugars or lipids.
This project is conducted in close collaboration to the CORAIL team glyco-chemistry and bioconjugates at the CEISAM laboratory (CNRS UMR6230 – David Deniaud and Sébastien Gouin).
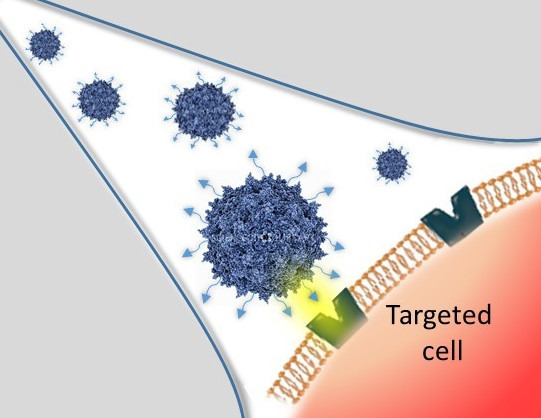
Project 1 - Chemical modification of amino group on the surface of the capsid of AAV
These modifications are achieved by chemical coupling of a ligand by the formation of a thiourea functionality between the amino group of the capsid proteins and the reactive isothiocyanate motif incorporated into the ligand. This strategy does not require genetic engineering of the capsid sequence. The proof of concept was first evidenced using a fluorophore (FITC). Next, we coupled the N-acetylgalactosamine ligand onto the surface of the AAV capsid for asialoglycoprotein receptor-mediated hepatocyte-targeted delivery. Chemically-modified capsids also showed reduced interactions with neutralizing antibodies. Taken together, our findings reveal the possibility of creating a specific engineered platform for targeting AAVs via chemical coupling.

Project 2 - Chemical modification of tyrosine group on the surface of the capsid of AAV
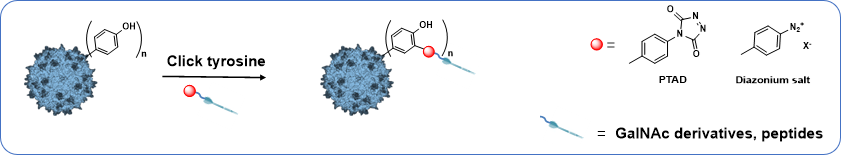
This project will investigate the possibility of re-targeting rAAV vectors via covalent linking of specific ligands to the tyrosine of AAV capsid by taking advantage of click-tyrosine chemical modification. Indeed it has been demonstrated that surface exposed tyrosine mutation on AAV2 capsids avoided degradation by the proteasome and resulted in high-efficiency transduction in human cells in vitro and murine hepatocytes in vivo. This “enhancer effect”, obtained after tyrosine mutation, has only been observed for this amino-acid and not after lysine mutation. These results demonstrated the prominent role of rAAV capsid tyrosine in cellular trafficking and transgene expression.
This part will focus on the bioconjugaison of click tyrosine ligand, having specific targeting properties, on the capsid of AAV in one step.
The proof of concept data has been protected by Patent filed in 2019.

2023 Team members :
Mathieu Mével, PhD, Senior scientist (Associate researcher, Corail Team - CEISAM laboratory UMR CNRS 6230)David Deniaud, Associate professor (Corail Team - CEISAM laboratory UMR CNRS 6230)
Mohammed Bouzelha, Engineer
Sébastien Gouin, CNRS Research Director (Corail Team - CEISAM laboratory UMR CNRS 6230)
Karine Pavageau, Engineer
Dimitri Alvarez-Dorta, Engineer
Sarah Renault, Engineer
Maia Marchand, PhD Student
Héloïse Delépée, Master degree student
With the growing success of gene therapy clinical trials, adeno-associated virus vector (AAV) manufacturing and quality control requirements tend to increase. Our group aims at improving current large scale viral vector production systems, such as the Sf9/Baculovirus expression system. We also develop novel analytical tools, based on next-generation sequencing (NGS), allowing us to identify and quantify the acid nucleic content of AAV particles.